Group Janssen
Dr. Aniek Janssen

Areas of expertise uitklapper, klik om te openen
Dr. Aniek Janssen started as a group leader in February 2019. She received her PhD (cum laude) in the lab of dr. René Medema (Dutch Cancer Institute) where she studied the consequences of chromosomal instability, a major hallmark of cancer, in both cells and mouse models. She also focused on designing ways to exploit chromosomal instability in anti-cancer strategies and developed an intravital imaging technique to visualize chemotherapeutic effects in tumor tissue
During her postdoc in the lab of dr. Gary Karpen (UC Berkeley/Lawrence Berkeley National Lab), Aniek studied how different chromatin domains within the nucleus respond to DNA damage. She developed locus-specific systems that allow for the inducible generation of single double strand breaks in Drosophila tissues and cells. By using these models in combination with genetics, live tissue-imaging and computational analyses, she has been able to perform an in depth analysis of the 3D nuclear movements and repair of double-strand breaks within different chromatin domains.
Her group will focus on understanding the role of chromatin dynamics in safeguarding the genome. She will use multi-disciplinary approaches, integrating epigenetics with cell biology, biochemistry and computational biology.
Research program uitklapper, klik om te openen
Genetic instability is a major hallmark of cancer and causes the cells’ genetic make up to continuously change. One major player in the manifestation of genetic instability is DNA damage, which can be caused either by endogenous (e.g. DNA replication) or exogenous insults (e.g. UV light, X-rays).
The eukaryotic nucleus is a cellular compartment that is far from homogeneous. It contains a plethora of distinct chromatin domains, which differentially regulate the functions of the underlying DNA sequences. These chromatin domains are crucial to the normal development of organisms and are each associated with specific histone modifications that regulate gene activity as well as proper packaging of the DNA. Dramatic changes in the distribution of chromatin modifications as well as mutations in histones and histone modifier proteins are associated with tumorigenesis, and are thought to play a prominent role in cancer progression
Research projects uitklapper, klik om te openen
The Janssen group aims to understand how diverse chromatin domains, as well as cancer-specific chromatin changes, shape the cellular response to DNA damage, which could have important implications for understanding cancer development as well as treatment.
Chromatin domain-specific break systems to study DNA damage repair
Targeted systems that induce locus-specific DNA damage are an extremely useful tool for performing in depth analyses of the DNA damage response in various chromatin regions. We employ and develop new locus-specific DNA damage systems in both Drosophila animals and mammalian cells. We use these systems in combination with high-resolution live tissue imaging, in depth sequencing as well as genetics. Our goal is to understand how different chromatin regions influence repair pathway usage, repair kinetics and movement of damaged sites in the 3D nuclear space.
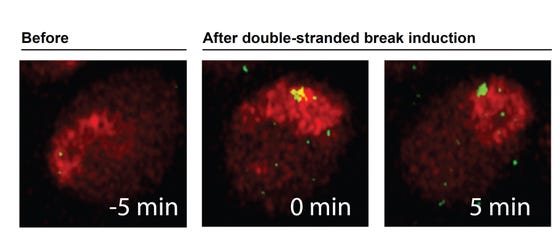
Targeted break system in Drosophila tissue at work. A single double strand break (green) was induced in the heterochromatin domain (red) of this cell. The double strand break moves to the periphery of the heterochromatin domain within 5 minutes in vivo.
The role of chromatin modifiers in DNA damage repair
Different chromatin regions display major differences in DNA compaction, histone modifications and biophysical properties. This indicates that different chromatin domains require different local chromatin changes at the damaged site to promote proper DNA damage repair. We aim to use high-resolution live imaging, chromatin assays, as well as mass-spectrometry based approaches to understand the role of damage-associated chromatin modifiers and histone modifications in repair.
Cancer-associated chromatin changes and genetic instability
Epigenetic changes and mutations in chromatin proteins are another major hallmark of cancer. However, how cancer-associated epigenetic changes can contribute to genetic instability remains largely unknown. Our long-term directions include the investigation of the effects of oncogenic chromatin changes on DNA damage repair, and to examine the potential of exploiting these changes in anti-cancer strategies. We will introduce these chromatin changes in human cells and organoids using CRISPR–Cas9 based assays. In addition to analyzing the effects of oncogenic changes on DNA damage repair, these engineered systems will be used to screen for synthetic lethal interactions in order to develop new anti-cancer therapeutics.
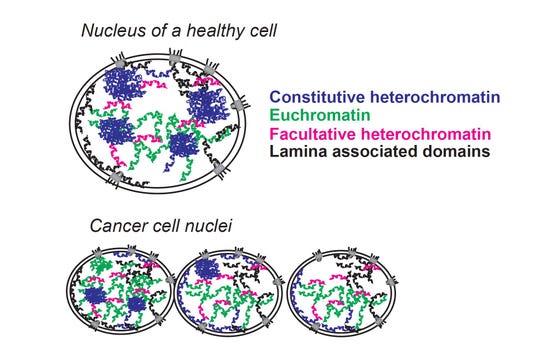
Chromatin changes in cancer cells. This simplified drawing of chromatin domains in nuclei exemplifies the major chromatin changes that cancer cells undergo .
Group members uitklapper, klik om te openen
- Apfrida Kendek - PhD student
- Severina Pociunaite - Master student
- Marieke Wensveen - PhD student
- Marit van Bueren - PhD student
- Jan Paul Lambooij - Senior research technician
Contact information uitklapper, klik om te openen
You can send an
LinkedIn
Visiting address:
Stratenum 2.131
Universiteitsweg 100
3584 CG Utrecht
The Netherlands
+31 88 75 50701
Key publications uitklapper, klik om te openen
- A. Janssen*, S.U. Colmenares*, T. Lee, G.H. Karpen. Timely double-strand break repair and pathway choice in pericentromeric heterochromatin depend on the histone demethylase dKDM4A. Genes & Development, (2019)
- A. Janssen*, S.U. Colmenares*, G.H. Karpen. Heterochromatin: guardian of the genome. Review. Ann. Rev. Cell. Dev. Biol., (2018)
- A. Janssen, G. Breuer, E. Brinkman, A. van der Meulen, S. Borden, B. van Steensel, R. Bindra, J. LaRocque and G. Karpen. A single double-strand break system reveals repair dynamics and mechanisms in heterochromatin and euchromatin. Genes and Development (2016).
- A. Janssen*, E. Beerling, R*. Medema** and J. van Rheenen**. Intravital FRET imaging of tumor cell viability and mitosis during chemotherapy. PLoS One (2013)
- A. Janssen, R. H. Medema. Review. Genetic instability: tipping the balance. Oncogene. (2013)
- A. Janssen, M. van der Burg, K.Szuhai, G.J.P.L. Kops, R.H. Medema, Chromosome segregation errors as a cause of DNA damage and Structural Chromosome Aberrations, Science (2011)
- A. Janssen, G. J. Kops, R. H. Medema, Elevating the frequency of chromosome mis-segregation as a strategy to kill tumorcells, Proc Natl Acad Sci U S A (2009).
* / **: these authors contributed equally
